Fanconi syndrome-associated interstitial lung disease
- 1 Department of Respiratory Medicine, University General Hospital of Patras, Patras, Periféria Dhitikís Elládh, Greece
- 2 Respiratory, University General Hospital of Patras, Patra, Greece
- 3 Department of Respiratory Medicine, University General Hospital of Patras, Patras, Greece
- Correspondence to Professor Argyrios Tzouvelekis; atzouvelekis@upatras.gr
Abstract
A middle-aged man was referred to our respiratory department with dyspnoea progressively deteriorating and non-productive cough over the past 8 months. High-resolution CT revealed multiple bilateral consolidations, traction bronchiectasis, reticular pattern and honeycombing with basal and peripheral predominance. Serology tests were negative. Pulmonary function tests showed moderate restrictive functional impairment and severe reduction in diffusing capacity for carbon monoxide. Meticulous evaluation of patient’s medical history revealed recent administration of oral corticosteroid due to pulmonary fibrosis potentially in the context of Fanconi syndrome diagnosed at childhood. The working diagnosis of interstitial lung disease (ILD) as a rare complication of Fanconi syndrome was proposed following multidisciplinary discussion. Despite combination treatment with low doses of corticosteroids and antifibrotic compound, the patient exhibited clinical, radiological and functional deterioration, was admitted to intensive care unit due to respiratory failure following infection-driven progression of fibrotic ILD and finally died.
Background
Fanconi syndrome is a rare global dysfunction of the proximal tubule leading to excessive urinary excretion of amino acids, glucose, phosphate, bicarbonate, uric acid and other solutes normally reabsorbed by the proximal tubule.1 The diagnosis of Fanconi syndrome is made based on tests documenting excessive loss of substances such as phosphate, amino acids, glucose, bicarbonate in the urine and the absence of high plasma concentrations in the respective substances. Urinary hyperexcretion of amino acids of all classes, evidence of type 2 renal tubular acidosis with metabolic acidosis or decreased serum phosphate on unrestricted dietary intake are among the most common signs that should fuel meticulous evaluation for Fanconi syndrome. Aetiology differs depending on the age of diagnosis. In childhood, inborn errors of metabolism consist of the principal cause, while in adults, the disease is acquired and usually caused by toxins or drugs.2 3 Treatment is not specific and mainly consists of replacement of substances lost in the urine.1 Pulmonary manifestations are not common in Fanconi syndrome, as progressive renal impairment resulting in metabolic acidosis, aminoaciduria, phosphaturia and proteinuria is the representative manifestation of the syndrome. Nevertheless, several familial cases of Fanconi syndrome may lead to both kidney and lung injury with relatively unknown pathogenic mechanisms.4
We report a case of interstitial lung disease (ILD) in a patient with Fanconi syndrome. Multidisciplinary discussion (MDD) between pulmonologists, nephrologists and internists suggested ILD as a rare complication of Fanconi syndrome. The following report presents in detail patient’s management and clinical course as well as highlights the cardinal role of MDD in diagnosis and management of rare causes of ILDs.
Case presentation
A middle-aged man, never smoker, presented to our department with rapidly progressive dyspnoea on exertion and non-productive cough over the past 8 months. Chest high-resolution CT (HRCT) revealed multiple bilateral consolidations, traction bronchiectasis, reticular pattern and honeycombing with basal and peripheral predominance. He denied the presence of fever, night sweats or weight loss. The patient reported a medical history compatible with Fanconi syndrome diagnosed almost 40 years ago, hypothyroidism and atrial fibrillation treated with ablation in 1997. The patient was receiving phosphate, potassium bicarbonate and cholecalciferol, since childhood as a replacement treatment for Fanconi syndrome and oral prednisolone as therapeutic option for ILD first identified in HRCT 12 months ago. The patient reported no occupational or environmental fibrogenic exposures.
Physical examination revealed the following vital signs: blood pressure of 135/70 mm Hg; heart rate, 67 beats/min; temperature, 36,7 °C and oxygen saturation, 97% on ambient air. Lung auscultation revealed bibasal end-inspiratory crackles (velcro type). Heart and abdomen examination was unremarkable. Clubbing was present. There were no palpable lymph nodes. Timeline of clinical features is depicted in figure 1.
Depiction of timeline of clinical features from childhood to adult life (created by Theodoros Karampitsakos).

Investigations
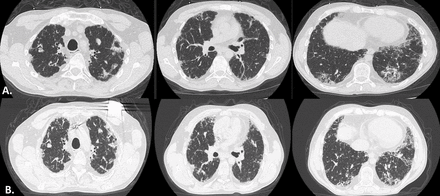
At the time of referral to respiratory department, HRCT revealed multiple bilateral consolidations, traction bronchiectasis, reticular pattern and honeycombing with basal and peripheral predominance indicative of indeterminate for Usual interstitial pneumonia (UIP) pattern (figure 2A). The complete blood and metabolic panel revealed renal impairment (serum creatinine: 3 mg/dL), while complete serology profile was negative. The patient did not undergo conventional bronchoscopy due to non-compliance and informed consent withdrawal. Pulmonary function tests (PFTs) showed moderate restriction and severe reduction in diffusing lung capacity for carbon monoxide (Forced vital capacity: 57%, Tiffeneau: 91, Total lung capacity: 65%, Diffusing capacity for carbon monoxide: 41%). Echocardiogram was normal with no signs of pulmonary hypertension.
(A) Chest CT of the patient receiving MMF plus corticosteroids for ILD as a complication for Fanconi syndrome showing bilateral consolidations, traction bronchiectasis and fine reticulation of subpleural distribution. (B) Radiologic progression 12 months after MMF discontinuation. ILD, interstitial lung disease; MMF, mycophenolate mofetil.
Differential diagnosis
On the basis of a compatible clinical (cough and dyspnoea on exertion), functional (restrictive pattern with diffusing capacity impairment) and radiological features (fibrotic pattern indeterminate for UIP) in the context of negative serology and absence of bacterial infection as well as familial history or major fibrogenic exposures, MDD work-up set a working diagnosis of Fanconi syndrome-associated fibrotic ILD.
Treatment
Following MDD, the patient was commenced on mycophenolate mofetil (MMF) (2 g/day) plus low doses of oral prednisolone (10 mg/day).
Outcome and follow-up
In a follow-up appointment after 6 months, the patient presented with no radiologic and functional improvement on HRCT and PFTs, respectively. MMF was discontinued on the basis of patient’s preferences and close monitoring was decided. Unfortunately, 6 months later, the patient presented with clinical, functional and radiological deterioration (figure 2B) and we commenced nintedanib in the context of progressive-fibrotic ILD (PF-ILD). Nintedanib was discontinued due to severe gastrointestinal disorders and excessive diarrhoeas leading to further renal function impairment. The patient remained at baseline supplementation treatment for Fanconi syndrome. Eight months later, the patient presented with fever, productive cough, worsening of dyspnoea and severe respiratory failure and was admitted to the intensive care unit, intubated and finally passed away. Thorough microbiology and virology investigation was negative. Overall course of PFTs is shown in figure 3.
Overall course of PFTs (created by Theodoros Karampitsakos). PFT, pulmonary function test; FVC, Forced vital capacity; DLCO, Diffusing capacity for carbon monoxide.

Discussion
Currently, there are only handful reports of Fanconi syndrome-associated ILD cases.4 5 Whole genome studies have recently revealed a variant associated with Fanconi syndrome and lung fibrosis.6–8 The denominated Acadian variant represents a variant found exclusively in Acadians, a founder population in Nova Scotia, Canada and is associated with abnormalities in proximal tubular reabsorption, chronic kidney disease and pulmonary fibrosis development. This rare variant fully segregated with the aforementioned phenotype in the expected autosomal recessive mode of inheritance. Whole exome and genome sequencing showed that this variant was located in intron 2 of NDUFAF6 (NM_152416.3; c.298–768 T > C) and led to mitochondrial respiratory chain complex I deficiency and defects in mitochondrial respiration.5 Impressively, besides Fanconi syndrome, recent seminal studies highlighted the emerging role of mitochondrial dysfunction and metabolic aberrations in the pathogenesis of pulmonary fibrosis.9 10 One limitation in our case report is that we were not able to test for a similar genetic mutation in the patient as that described in Acadians, yet our aim was to highlight this rare coexistence. Cystinosis as potential genetic cause of Fanconi associated with pulmonary manifestations in later life was excluded as in this disease pulmonary complications are related with respiratory muscle dysfunction and not parenchymal lung disease.11 An association between the two diseases has not been proven in this patient.
Based on the above, there is a pressing need for registries of Fanconi syndrome—associated ILD cases in Europe aiming to endotype patients and potentially identify novel variants. Genetic counselling as part of the MDD approach in these patients is strongly encouraged. Our case report highlights the need for early referral of patients with Fanconi syndrome and progressive dyspnoea from nephrologists to pulmonologists for extensive functional and radiological work-up. Increasing awareness is an important step for early diagnosis and optimal management as these cases may develop a progressive fibrotic phenotype and may benefit from timely interventions including immunomodulatory and antifibrotic compounds.
Learning points
-
This is a rare case of interstitial lung disease in a patient with Fanconi syndrome.
-
A mutation in complex I assembly factor NDUFAF6 leads to mitochondrial respiratory chain complex I deficiency and this may lead to remodelling and fibrosis.
-
Early referral of patients with Fanconi syndrome and progressive dyspnoea from nephrologists to pulmonologists for extensive functional and radiological work-up is important.
-
Increasing awareness is an important step for early diagnosis and optimal management as these cases may develop a progressive fibrotic phenotype and may benefit from timely interventions including immunomodulatory and antifibrotic compounds.
Ethics statements
Patient consent for publication
Footnotes
-
Contributors OP, TK, FS and AT contributed substantially to the conception and design of the work. OP and TK organised and entered data. OP, TK and AT wrote the main draft of the manuscript. OP, TK, FS and AT revised the case report for important intellectual content. All authors contributed to the final drafting of the manuscript.
-
Funding The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
-
Case reports provide a valuable learning resource for the scientific community and can indicate areas of interest for future research. They should not be used in isolation to guide treatment choices or public health policy.
-
Competing interests None declared.
-
Provenance and peer review Not commissioned; externally peer reviewed.
- © BMJ Publishing Group Limited 2022. No commercial re-use. See rights and permissions. Published by BMJ.
References
Use of this content is subject to our disclaimer